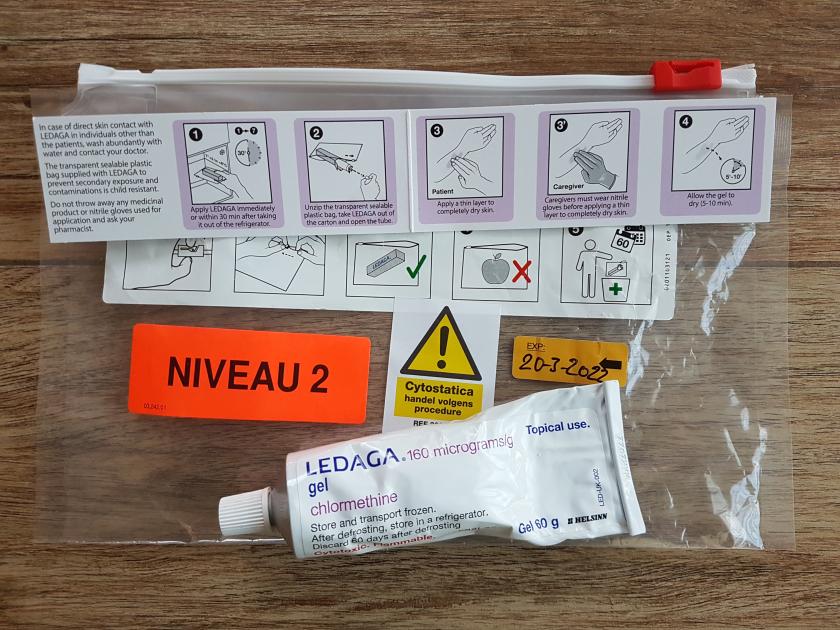
Actelion is Granted Marketing Authorization by the European Commission for Ledaga (Chlormethine Gel) for the Treatment

Juniper Biologics signs exclusive license agreement with Helsinn Healthcare SA for LEDAGA® (chlormethine) in Australia, Asia and the Middle East